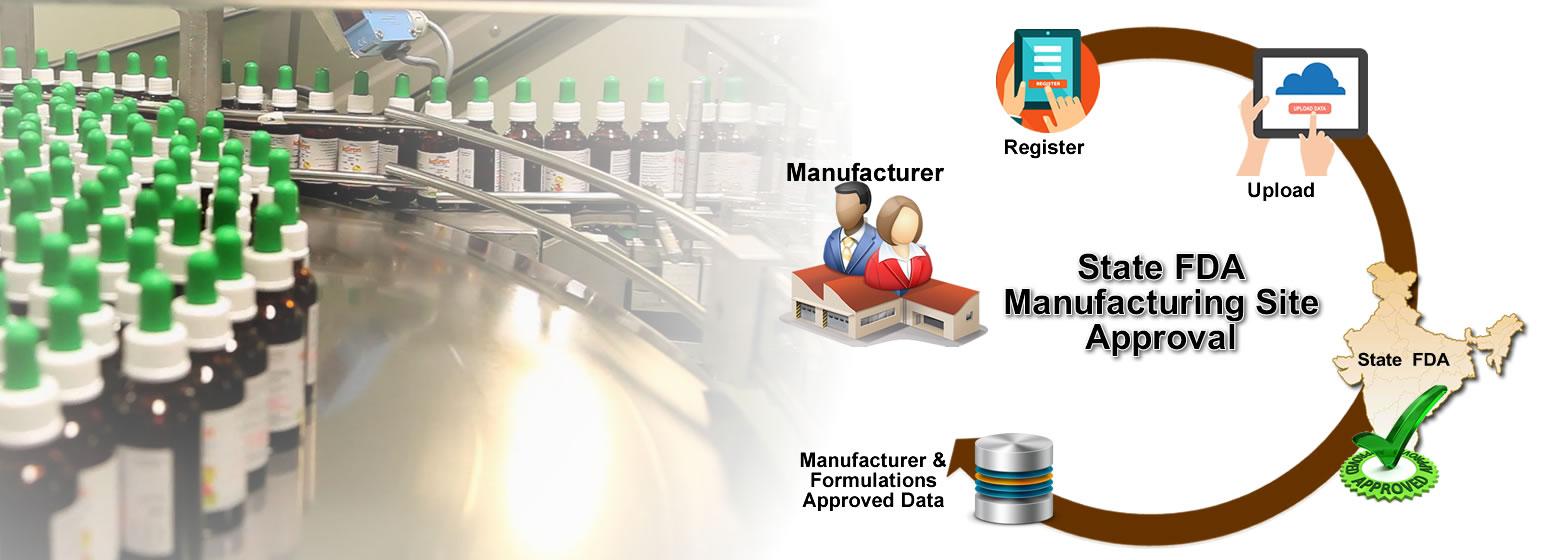
22.0 Information required to be submitted for the in vitro diagnostic medical device: ...
Accredited Consultants Pvt Ltd 2023-10-05T05:34:31 2023-10-05T05:34:31
Accredited Consultants Pvt Ltd 
22.0 Information required to be submitted for the in vitro diagnostic medical device: (1) The detail